Tag Archives: Viral Hepatitis
Dr. Thomas is helping lead the HCV Elimination Program in Florida.
The FL HCV elimination group convened national and local experts in August 2019 in Orlando, FL for its inaugural meeting to address screening, treatment-level barriers and liver cancer prevention. The meeting goal was to support a statewide HCV elimination program and it was attended by stakeholders including representatives from the FL DOH, patients, medical and social service providers, researchers, policymakers and others from private organizations. This followed an earlier HCV elimination meeting in February 2019, held in Miami, FL by the major liver disease societies (AASLD/EASL). Planned activities will strengthen and be synergistic with the current efforts from the FL DOH viral hepatitis program and include involvement of four major academic centers in FL. Stakeholders will continue to meet in 2020 with the goal of producing a guidance document with support from both the American Liver Foundation and ASTHO by participating in monthly peer-to-peer learning opportunities for hepatitis elimination.
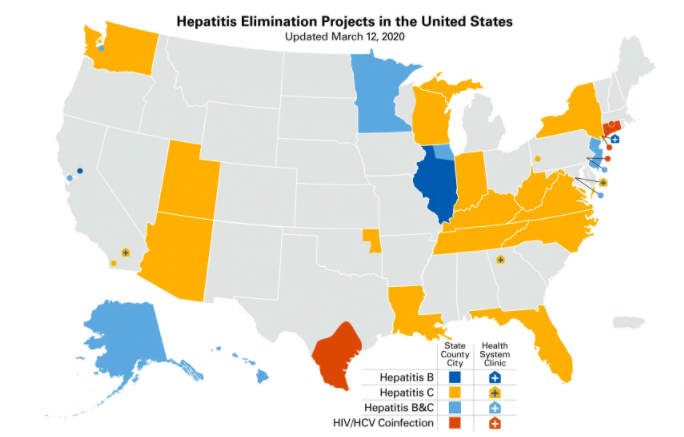
Click here for more information about the Hepatitis Elimination Project
To participate in the State of Florida, please contact:
Thomas Bendle email: Thomas.Bendle@flhealth.gov
Emmanuel Thomas email: ethomas1@med.miami.edu
The University of Miami Miller School of Medicine was awarded the Gilead Sciences “FOCUS Grant” to support identifying HIV and Hepatitis C infected patients, HIV and Hepatitis C testing, and testing-related services.
The University of Miami Miller School of Medicine has been awarded a $299,220 grant from Gilead Sciences, Inc., to develop a replicable model program that embodies best practices in HIV and/or hepatitis screening and linkage to care. The award is provided through Gilead’s Frontlines of Communities in the United States (FOCUS) Program. The grant application described that a growing body of research has shown that opt-out testing can play a strong role in getting more individuals tested, extending earlier and better care to people who have been infected, improving their quality of life and promoting better disease management. The FOCUS program will support local initiatives to identify and link to care patients infected with HIV and hepatitis C (HCV) at the University of Miami Miller School of Medicine Emergency Room while developing and promoting a replicable model program that embodies best practices in HIV and HCV.
Dr. Dushyantah Jayaweera M.D., Senior Associate Dean for Research said, “We are thankful to Gilead for this grant, which will enable UM to better serve patients and improve their quality of life. The Centers for Disease Control and US Preventive Services recommendations, backed by this key support from Gilead, will help our caregivers to more effectively address the health and wellness needs of these patient populations throughout South Florida. We are thankful to Gilead for this grant, which will enable UMMSM to better serve patients and improve their quality of life. The Centers for Disease Control and U.S. Preventive Services recommendations, backed by this key support from Gilead, will help our caregivers to more effectively address the health and wellness needs of these patient populations throughout South Florida.”
Dr. Emmanuel Thomas M.D. said, “We are grateful to Gilead for granting these funds. These dollars will enable us to develop a program structured to deal with the current gap in care caused by the inability to identify HIV and Hep C-infected individuals. We hope to extend this funding to include patients with Hepatitis B in the near future.”
As described in the Gilead grant application, “a growing body of research shows that opt-out testing can play a strong role in getting more individuals tested, extending earlier and better care to infected individuals, improving quality of life, and promoting better disease management that reduces new infections. In light of the 2006 Centers for Disease Control (CDC) recommendations for routine opt-out HIV testing, the 2012 CDC recommendations for HCV screening, the US Preventive Services Task Force recommendations for routine HIV and HCV screening, and other recognized best practices in HIV and HCV screening”.
ALIGNMENT WITH NATIONAL EFFORTS
In light of the 2006 Centers for Disease Control (CDC) recommendations for routine opt-out HIV testing, the 2012 CDC recommendations for HCV screening, the U.S. Preventive Services Task Force (USPSTF) recommendations for routine HIV, HCV and HBV screening, and other recognized best practices, UMMSM will work to develop and promote a replicable model program that embody uses these recommendations and best practices in screening and testing for the viruses.
The rates of HCV infections are climbing in the U.S., according to the Centers for Disease Control and Prevention (CDC), with many health professionals describing the increase as an emerging public health crisis. Research suggests that the increase in HCV could be linked to the increase of injection drug use throughout the country.
ALIGNMENT WITH LOCAL EFFORTS
Emmanuel Thomas, M.D., Ph.D., FAASLD was recently invited to give an oral presentation, based on the abstract titled, “Hepatitis C Virus sustained virological response in the real-world uninsured patient population treated with direct acting antivirals,” at the annual American Public Health Association meeting in San Diego.
Because Florida has been hit hard by the opioid epidemic and Miami has one of the highest new HIV infections in the USA, identifying patients with HIV and hepatitis C is crucial to improving the overall health of the state. Testing at the University of Miami Miller School of Medicine is underway throughout the medical school.
“The FOCUS partnership, in many ways, is our Medical School’s vision in action – bolstering the standing of the our institution as a community partner, while directly benefiting South Floridians,” Dr. Jayaweera professor in the Department of Infectious Diseases and a principal investigator on the FOCUS grant, said. “We leveraged departmental expertise and infrastructure to win this competitive grant, and in doing so, we have already increased our Emergency Department-based HIV and HCV testing significantly. With this ramped up testing, we have enhanced the care of South Floridians through increased detection of the diseases and linkage to care.” Dr. Jayaweera noted the importance of testing to inform those who did not know they were infected or at risk and the difference it can make in their life. “Currently, South Florida ranks first in the nation in the rate of new HIV infections,” he said. “A substantial proportion of these infections are acquired through injection drug use and needle sharing, which increases concern over possible co-infection with HCV. In addition to helping prevent disease transmission through early identification, our program also links patients to care, which is now more important than ever given that HCV is largely curable, and HIV can be managed as a chronic condition. Ultimately, we believe that scaled-up, electronic medical record-based screening and linkage to care will reduce morbidity and mortality associated with HCV and HIV in both Miami and South Florida grappling with the current opioid epidemic,” he said.
ABOUT GILEAD
Gilead Sciences, Inc. is a research-based biopharmaceutical company that discovers, develops, and commercializes innovative medicines in areas of unmet need. As a leader in developing therapies for HIV and chronic hepatitis C virus infection, Gilead is committed to helping ensure access to life-saving screening and care services for people who could benefit from them.
The biopharmaceutical company Gilead researches, develops, manufactures, and markets human pharmaceuticals for certain diseases, including the hepatitis C virus (HCV), hepatitis B virus (HBV) and the Human Immunodeficiency Virus (HIV). Gilead will provide these funds to UMMSM through its Frontlines of Communities in the United States (FOCUS) Program to financially support these efforts.
ABOUT FOCUS
FOCUS is an initiative of Gilead Sciences, Inc., which was created in 2010 to address the issue of HIV/AIDS transmissions and to inform those who did not know they were infected or at risk. In 2013, FOCUS also added hepatitis C testing per recommendation from the CDC and by the United States Preventive Services Task Force. The main goal was to make HIV and HCV screening a standard of care and to change public perceptions as well as overcome stigma that may have discouraged this type of testing.
Gilead launched the FOCUS Program in 2010 to address one of the most pressing problems driving HIV and acquired immunodeficiency syndrome (AIDS) transmissions. At the time, it was estimated that one in five HIV-positive Americans, or approximately 230,000 people, didn’t know they were infected. FOCUS was further expanded in 2013 to include HCV, and again in 2015 to include HBV, as a result of both the CDC and the USPSTF recommendations for testing those viruses. Today, FOCUS is a partner of more than 100 healthcare institutions, government agencies and community stakeholders to make routine HIV, HCV and HBV screening a standard of care practice, to reduce the number of undiagnosed individuals, to decrease the number of late diagnoses and ensure strong linkage to care, to expand open dialogue about the viruses, and to change public perceptions and overcome stigma.
In 2010, Gilead Sciences, Inc. launched the FOCUS program (On the Frontlines of Communities in the United States) to address one of the most pressing problems driving human immunodeficiency virus (HIV) and acquired immunodeficiency syndrome (AIDS) transmissions. At the time, an estimated one in five HIV-positive Americans, or approximately 230,000 people, did not know that he or she was infected. FOCUS was enhanced in 2013 to address Hepatitis C (HCV) and again in 2015 to address Hepatitis B (HBV) as a result of both the Centers for Disease Control and Prevention (CDC) and the United States Preventive Services Task Force (USPSTF) recommendations for HCV and HBV testing. FOCUS partners with over 100 healthcare institutions, government agencies and community partners to: make routine HIV, HCV, and HBV screening a standard of care for appropriate patient populations; reduce the number of undiagnosed individuals, decrease the number of those who are diagnosed late and ensure strong linkage to care; expand stakeholder dialogue on these issues; and change public perceptions and overcome stigma that may discourage testing.


Dr. Thomas has received a $2 million dollar grant from the FL Department of Health to study virus infection and progression of liver disease to liver cancer.
The Florida Department of Health has awarded Dr. Thomas the Bankhead-Coley Florida Clinical Cancer Research Grant to lead a research project titled “Identifying Infection and Molecular Determinants of Health Disparities in HCV Infected Minority Populations for the Prevention of HCC”. The project is designed to address health disparities in minority populations with HCV-attributable liver disease in order to increase the early detection of HCC. The study aims to aid in the detection of high-risk subgroups and improve understanding of the progression of HCV disease after infection.


Dr. Thomas has received a $2 million dollar grant from the NIH to study acute & chronic viral infections.
The NIH funded project titled “A Multifaceted Approach to Study Tissue and Cell Type Specific Molecular Mechanisms of the Host Response to Acute/ Chronic Viral Infection” is lead by Dr. Thomas and awarded to the University of Miami School of Medicine. The project is intended to elucidate the tissue specific molecular mechanisms that drive disease progression in patients with chronic viral infections, as these patients are susceptible to high morbidity and mortality rates due to progressive organ damage. The project findings would subsequently be used to identify new targets for therapeutic intervention.
Very few viruses are able to manifest as chronic infections in humans. The intrinsic innate immune response provides a first line of defense against invading viruses; however, in the case of chronic viral infections, these initial responses that were ineffective at controlling virus replication can then cause disease over many years due to chronic activation. In most organs, epithelial cells are some of the first cells to encounter viruses in the human body and innate immune responses in these cells are paramount to driving subsequent immune control. Interestingly, epithelial cells predominantly produce type III interferons (IFNs) in response to viral infection whereas immune cells produce Type II IFNs (γ) and Type I IFNs (α/β) are produced by most cells in the body. The mechanism underlying cell type and tissue specific expression of the type III IFNs are unknown and likely involve regulation of epigenetics modifications, gene expression of pattern recognition receptors and associated signaling molecules. In drosophila, the fat body is the primary innate immune organ producing antimicrobial peptides in response to pathogens. The human liver, equivalent to the drosophila fat body in terms of function, utilizes Type III IFN responses to fight viral infection and likely possesses other unique properties with respect to innate immunity when compared to other organs.
We have developed novel and exciting in vitro models that utilize primary epithelial cells from several organs that have intact innate immune responses when compared to immortalized or transformed cell lines. We and others have shown that these cell types are of critical importance in the development of disease since they directly detect components of viral pathogens. We therefore assert that primary cells are the optimal model to use for studies on innate immunity and we propose a novel approach to study innate immunity based on the innate immune pathways that we have demonstrated to be important for microbial pathogenesis. In addition, we are developing novel physiologic models incorporating primary epithelial cells, stem cell-derived epithelial cells, 3-dimensional chip and microfluidic-based platforms.
The use of stem cell-derived cells would facilitate the identification of changes in gene expression, which occur during differentiation, that contribute to the unique innate immune system in epithelial cells. The specific goals of this program are to functionally characterize the innate immune response, including the production of Type III IFNs, to multiple viral pathogen associated patterns, including both DNA and RNA sensing pathways, and to elucidate the underlying molecular mechanisms through which innate immunity manifests in epithelial cells using sophisticated in vitro models. In addition, tissue specific and developmental expression of specific innate immune signaling components including, TLR3, STING and IRF7, will be addressed as a mechanism underlying tissue specific responses. Completion of these studies would offer the most in depth characterization of innate immunity in epithelial and other cell- types while improving our understanding of its contribution to human disease in multiple organs.


Dr. Thomas Receives Travel Award to HIV and Liver Disease meeting 2016.
Liver disease has emerged as a major cause of morbidity and mortality in individuals infected with HIV, including those infected with HBV and HCV. The HIV & Liver Disease 2016 conference is designed to address the increasingly prevalent clinical issues and challenges associated with HIV and liver disease,











